Methodology from Smith et al (2011):
As a part of the Interannual Variability in the Antarctic-Ross Sea (IVARS) program, two McLane Mark 78-H PARFLUX time-series sediment traps were deployed on moorings during the austral summers of four years: 2001-2002, 2003-2004, 2004-2005, and 2005-2006. Moorings were deployed in late December (late spring) in ice-free waters and retrieved in early February (summer) using either the U.S.C.G.C Polar Star or the R.V.I.B. Nathaniel B. Palmer (Table 1 provides the deployment and recovery data) at 77°S, 172.7°W (Callinectes) and 77.8°S, 180° (Xiphius) in the southern Ross Sea (Fig. 1). Water depth for both moorings was approximately 600 m. The sediment traps were moored at 200 m, and each mooring had a long "S" tether to decouple the two sections of the mooring and to place the shallow sensors at relatively fixed depths relative to the surface (Fig. 2). All traps were baffled. Additionally, InterOcean Systems S-4 current meters were deployed 5 m above each trap, and current speeds and directions were binned and recorded every 5 min. Tidal signals were strong, and 12 h means calculated and analyzed. Each sediment trap cup collected for an equal time period based on the date of deployment and the anticipated date of recovery - between 2 and 3 d. Before deployment, each sample cup was filled with preservative solution that consisted of buffered 2% formalin and 50 g L-1 NaCl final concentration (Asper and Smith, 1999). Data from the near-surface instruments are reported elsewhere (Smith et al., 2010). In all trap deployments, the mechanisms performed properly; however, a few samples were lost due to damage in shipment and a misalignment of the starting position on the sample carousel for Callinectes in 2005-2006.
After recovery, the preserved samples were returned to the laboratory and filtered through a 600 µm mesh to remove all the zooplankton swimmers before splitting. The mesh was rinsed into a glass vial and any remaining organisms were removed. Next, each filtered sediment trap sample was split into four samples using a four-way plankton splitter, with aliquots collected for total mass flux (TMF), biogenic silica (BSi), particulate organic carbon and nitrogen (POC and PN), and fecal pellet microscopy. Total mass flux (dry weight) samples were filtered through pre-weighed 0.8 µm Poretics polycarbonate filters, rinsed with ammonium formate to remove seawater salts, dried (at 60 °C for approximately 4 d, until a constant weight was obtained), and reweighed. POC and PN samples were filtered through precombusted Whatman GF/F filters and rinsed with ca. 5 mL 0.01 N HCl in seawater to remove inorganic carbon. Filters were then placed in combusted glass vials, covered with combusted aluminum foil, and dried at 60 °C. Once dry, POC/PN samples were processed on a Carlo-Erba 1108 elemental analyzer using acetanilide as a standard (Asper and Smith, 1999). Biogenic silica (BSi) concentrations were determined by filtering samples through 0.6 µm Poretics polycarbonate filters, placing the filters in plastic petri dishes, and drying at 60 °C. Filters were digested and quantified spectrophotometrically (Brzezinski and Nelson, 1989). Total mass flux, organic carbon and nitrogen, and biogenic silica were converted to flux by
(1) F=M/TA
where F is the flux (in weight or molar units m-2 d-1), M is the variable in question, A is the area of the sediment trap opening (0.5 m2), and T is the time interval in days when each cup remained open.
Aliquots for microscopy were further split using a smaller two-way Folsom plankton splitter until approximately 100 fecal pellets per sample were left for enumeration. Samples were then concentrated onto a 53 µm sieve; minipellets (Accornero and Gowing, 2003) were not included in the microscopic analyses. The samples were placed in clean petri dishes and imaged using an Olympus stereo dissecting microscope. Images of fecal pellets for 4-5 samples of selected trap intervals in each year were analyzed using Image Pro software, from which the length and width of each individual pellet were measured. Observations, including morphology and color, were taken from ca. 4000 pellets. Fecal pellet carbon was measured using the volumes calculated from microscopy (Kelchner, 2005) and volume to carbon relationships from the literature. Fecal pellet carbon values were not available from the Ross Sea; therefore, the lowest literature value used for other polar studies (0.016 mg C mm-2, euphausiid pellets from the Scotia Sea; González, 1992) and a middle range value (0.05 mg C mm-2, copepod pellets from Norway; Wexels Riser et al., 2002) were used to delineate the error surrounding these measurements (Gowing et al., 2001). All weights were converted to molar units for comparison with particulate organic carbon fluxes.
To assess net community production and estimate export, we constructed elemental budgets of nitrogen and silica in a manner similar to that of Bates et al. (1998), Smith and Asper (2000) and Sweeney et al. (2000). Because two periods were sampled (at the deployment and recovery of moorings), the temporal dynamics of nutrient uptake could be more finely resolved than in previous studies and related more closely to assemblage composition. Properties of the water column were sampled upon deployment and recovery of the moorings. CTD casts were completed immediately after mooring deployment using a SeaBird 911+ CTD mounted on a rosette fitted with 10 L Bullister bottles. We sampled twelve depths in the upper 200 m, with at least 75% of these concentrated in the euphotic zone. Nutrient (NO3, NH4, PO4, and Si(OH)4) samples were collected, filtered through 0.4 µm Acrodisc filters, and frozen. All nutrient concentrations were determined using automated techniques in the laboratory (JGOFS, 1996). Particulate organic carbon/nitrogen and biogenic silica concentrations were determined from seawater samples of known volumes that had been filtered through combusted GFF filters or polycarbonate filters (0.6 µm Poretics) and analyzed as described above. Water column values were calculated using trapezoidal integrations.
Production of PON [delta(NO3-)] was estimated from the seasonal uptake of nitrate at each station by
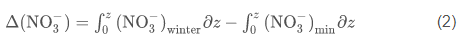
where z is the depth, and the subscripts min and winter refer to the observed nitrate concentrations in the water column and the winter nutrient concentration, respectively. Nitrogen units were converted to carbon units using the measured molar C/N ratio of particulate matter (Smith et al., 2006); all integrations were from 0 to 200 m. An integration depth of 200 m was chosen because this is far below the depth of nutrient removal during austral summer, and flux to greater depths can be considered to be "lost" from the surface layer, on at least seasonal time scales. Diffusional inputs through the strong pycnocline are ignored, as is horizontal advection, which appears to generate rapid changes in parameters but with no net changes on a seasonal basis (Smith et al., 2010). Similarly, the production of biogenic silica [delta(Si(OH)4)] was estimated using
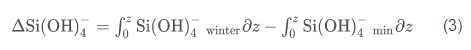
and converted to carbon units using the molar C/Si ratio (1.61) measured by Nelson and Smith (1986) for blooms in the Ross Sea overwhelmingly dominated by diatoms. There are no data to suggest that winter values of silicic acid and nitrate in the Ross Sea change on decadal or shorter time scales, and vertical mixing during winter and slow nutrient regeneration during the austral winter make nutrient concentrations nearly uniform throughout the water column (Gordon et al., 2000). Hence, winter nitrate and silicic acid concentrations can be reliably predicted from previously collected data (e.g., nitrate values=31.0 µM when normalized to S as 35 psu, and silicic acid values=80 µM; Smith and Asper, 2000; http://usjgofs.whoi.edu/jg/dir/jgofs/southern/). Nitrate potentially can be remineralized within the growing season via nitrification, but this process is extremely slow at the low temperatures of the Ross Sea and was ignored (Karl et al., 1996). Integrated NH4 concentrations are less than 5% of the total inorganic nitrogen concentrations at all times, and are also ignored for these calculations (Smith et al., 2006).
The export of particulate nitrogen and silicon was estimated by comparing the seasonal changes in their concentrations within the water column:

where E200 is the export from the upper 200 m, Prodi is the production of either N or Si at time i (estimated from Eqs. (2) and (3)), and PMi is the particulate nitrogen or silica concentration at time i. Particulate matter levels at the bloom's start are based on early spring measurements (http://usjgofs.whoi.edu/jg/dir/jgofs/southern/). We realize that for nitrogen some portion of the material may have entered the dissolved organic nitrogen (DON) pool, but we have no way to assess that pathway. All assumptions are discussed fully in Smith and Asper (2000) and Smith et al. (2006). Phosphate was not treated in this analysis due to a more limited particulate phosphorus data set.

 ©2024 Biological and Chemical Oceanography Data Management Office.
©2024 Biological and Chemical Oceanography Data Management Office.
