All samples used in this work were collected as part of the North Atlantic long-coring expedition in Oct.-Dec. 2014 (R/V Knorr, Cruise KN223); this project focuses on sediments from 4 sites (2, 3, 11, 12) exhibiting variations in the depth to which oxygen penetrates. The sediment subsamples were collected from long piston cores or shorter gravity cores. While oxygen penetrates through the full long core depth at sites 11 and 12, oxygen was consumed in the sediment column at site 3 and especially at site 2. All samples were collected anaerobically in order to perform on-board culture enrichments via the most probable number (MPN) method. Sediments were placed in sterile serum vials, capped with butyl rubber stoppers and flushed with N2 for 2 min and maintained at 4 degrees C for immediate shipboard MPN inoculation work (this dataset). Parallel samples were similarly collected from these and additional core sections and maintained at 4 degrees C for later determination of microbial production rates (see microbial production dataset).
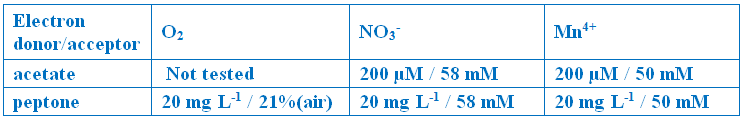
Twenty ml of anaerobic saline media was added to the 30 ml sediment within each serum vial and mixed to create a sediment slurry. MPN assays were initiated on-board and were designed to quantify the abundance of viable microbial cells with specified metabolisms. Hungate tubes with synthetic marine base salts media were amended with various combinations of electron donors (acetate, peptone) and acceptors (oxygen, nitrate, and manganese(IV) oxide):
 Saline basal medium was composed of (g/L): 0.2 NH4Cl, 30 NaCl, 2.8 MgCl2, 0.33 KCl, 0.3 CaCl2, 0.3 KH2PO4, 0.01 NaBr, 0.015 H3BO3, 0.02 SrCl2, 0.02 KI, 0.02 FeCl3, 0.0075 MnSO4, 0.0045 Na2WO4.2H2O, 0.003 NiCl2, 0.02 CoSO4, 0.0015 ZnSO4, 0.002 CuSO4, and 0.0015 Na2MoO4. pH was adjusted to 6.2.
Saline basal medium was composed of (g/L): 0.2 NH4Cl, 30 NaCl, 2.8 MgCl2, 0.33 KCl, 0.3 CaCl2, 0.3 KH2PO4, 0.01 NaBr, 0.015 H3BO3, 0.02 SrCl2, 0.02 KI, 0.02 FeCl3, 0.0075 MnSO4, 0.0045 Na2WO4.2H2O, 0.003 NiCl2, 0.02 CoSO4, 0.0015 ZnSO4, 0.002 CuSO4, and 0.0015 Na2MoO4. pH was adjusted to 6.2.
In the case of anaerobic MPN assays, Hungate tubes and their contents were boiled and purged with high purity N2 for 30 min. Aerobic assay tubes were prepared in air and were not purged. MPN assays were inoculated with 1 ml of sediment slurry and were diluted using 10-fold dilutions. MPN assays were incubated for 6-12 months at room temperature and were then assayed for activity/growth. Activity in MPN assays was evaluated by determining the headspace concentration of CO2 using an infrared gas detector. Additionally, colorimetric approaches specific to each anaerobic metabolism were used: the azo dye method (Strickland and Parsons 1968, Bull Fish Res Board Can 167:71) to detect the reduction of nitrate to nitrite and the T(4-CP)P method (Madison et al. 2011, Talanta, 84:374) to quantify production of Mn(II) from Mn(IV). Blank tubes were similarly prepared but not inoculated; these were analyzed to establish background levels of metabolites.
MPN assays were successful for all incubations using oxygen, Mn(IV) or nitrate as electron acceptors. However, the Mn(II) assay suffered from unidentified interferences in many cases. Growth as identified by CO2 accumulation was used where results of the Mn(II) assay were ambiguous. MPN results were converted to estimates of viable cell concentrations as follows. The highest dilution exhibiting evidence of growth was multiplied by the initial 1.6667-fold dilution used to make the sediment slurry. This count was considered the minimum MPN; the next higher dilution was considered the maximum MPN. For example, an assay exhibiting growth at 10^3 dilution but not at 10^4 dilution was considered to have a viable cell concentration of between 1,667-16,667 cells per cm^3 of sediment. For any subsequent calculations, such as cell turnover, the geometric mean of these values was used; e.g., 52,705 cells per cm^3 in this example case. In cases where no growth occurred at the lowest dilution or positive growth occurred at the highest dilution, MPN can only be constrained to be lower or higher than these estimates, respectively, and the MPN column is left blank.
For each electron acceptor/donor combination from each core section, the highest dilution MPN tube exhibiting growth was targeted for further culture transfers and eventual microbial identification/isolation. We have noted where PCR products were successfully obtained from these tubes, providing additional validation of the corresponding MPN result.

 ©2024 Biological and Chemical Oceanography Data Management Office.
©2024 Biological and Chemical Oceanography Data Management Office.
