Lead (Pb) isotope ratios from the U.S. GEOTRACES EPZT cruise (GP16, TN303) on R/V Thomas G. Thompson in the tropical Pacific from October to November 2013
Project
Program
| Contributors | Affiliation | Role |
|---|---|---|
| Boyle, Edward A. | Massachusetts Institute of Technology (MIT-EAPS) | Principal Investigator |
| Rauch, Shannon | Woods Hole Oceanographic Institution (WHOI BCO-DMO) | BCO-DMO Data Manager |
Abstract
The towed-fish sampling method used was exactly the same as the description in section 2.1 of the GEOTRACES cookbook in a positive-pressure filtered air environment filtered through cleaned 0.2 um Acropak capsule filters (Acropak filters were used for at most 3 stations before a new filter was used, and they were stored empty in a refrigerator while not in use) into warm-acid leached Nalgene 2-liter polyethylene bottles with polypropylene caps, rinsed 3 times with the sample. Bottles were then capped and not further handled until returned to MIT a few months later, where they were acidified to pH 2.0 with high-purity HCl and allowed to sit for a few months before analysis.
Analytical Procedures:
2L high-density polyethylene sample storage bottles with polypropylene caps and their top threads were soaked overnight in 2N reagent grade HCl, then filled with 1N reagent grade HCl to be heated in an oven at 60˚C overnight, inverted, heated for a second day, and rinsed 5X with pure distilled water. The bottles were then filled with trace metal clean dilute HCl (~0.01N HCl) and again heated in the oven for one day on either end. Clean sample bottles were emptied, rinsed with distilled water, and double-bagged prior to shipboard rinsing and filling with sample.
Samples were analyzed at least 1 month after acidification during 7 mass spectrometry sessions by a modified method based on that described Reuer et al. (2003) as modified by Boyle et al. (2012) and further slightly modified as noted in the following.
The isotope method begins with Nobias Chelate PA1 preconcentration followed by anion exchange purification. The PA1 method was used in preference to the Reuer et al. (2003) Mg(OH)₂ method because high Si levels in the Pacific Deep water (which are scavenged by Mg(OH)₂) lead to precipitation of silica gel upon dissolution (preventing column passage). The method starts with a two repeated batch ion-exchange chelation preconcentrations followed by the Reuer et al. (2003) anion exchange purification and isotope ratio analysis on a GV/Micromass IsoProbe multicollector ICPMS using a 50 uL/min nebulizer aspirated into an APEX/SPIRO desolvator, using post-desolvator trace N₂ addition to boost sensitivity.
Nalgene polypropylene separatory funnels (1000mL) and Corning 50 mL conical centrifuge vials were cleaned by heated submersion for 2 days at 60˚C in 1M reagent grade HCl, followed by a bulk rinse and 4X individual rinse of each vial with pure distilled water. Each funnel and vial was then filled with trace metal clean dilute HCl (~0.01M HCl) and heated in the oven at 60˚C for one day on either end. Separatory funnels and centrifuge vials were kept filled until just before usage. The separatory funnels were rinsed with distilled water after each use and then filled with high-purity distilled water spiked with high-purity HCl (final concentration ~0.01M) between uses.
Nobias Chelate PA1 resin was cleaned with 2 methanol rinses, and a distilled water rinse followed by leaching with ultrapure 6M HCl for 12-24 hours. This procedure was repeated twice, followed by two one-day leaches with ultrapure 3M HNO3 on a shaker table. The resin was then rinsed six times with distilled water to remove the nitric acid. It was then leached twice with ultrapure 0.1N HNO₃ for one day each. The final 0.1N rinse was checked for Pb blank by ICPMS and the resin only used if the blank was acceptably low. Because of the large amounts of resin used, the used resin from each sample was saved and re-washed using the following protocol: leach with 3M HNO3 for 1 day, rinse 4x with dH₂O, rinse with 0.1M HNO₃ for 2 days, rinse with fresh 0.1 M HNO₃ for 3 days, and then then rinse with dH₂O until the pH is ~5.
One or two 1000mL polypropylene separatory funnels (Nalgene) were weighed and rinsed one time with seawater sample, then filled with ~1000 mL (with up to 700 mL in the second funnel) of sample. The pH of the solution was adjusted by addition of purified ammonium hydroxide/acetic acid pH=7.98 buffer (to a final pH>4; preferably pH~4.1 to keep the buffer blank low). A pre-cleaned aliquot of Nobias Chelate PA1 resin was added, and agitated on a shaker table for one day. Then the resin was allowed to settle to the bottom of the separatory funnel and drawn off into a 50 mL centrifuge tube. A second batch of Nobias Chelate PA1 resin was then added and agitated for one day on a shaker table. Then the second batch of resin was allowed to settle to the bottom of the separatory funnel and drawn off into the same 50 mL centrifuge tube. The solution/resin mix was centrifuged and the supernatant solution siphoned off. Pb was released from the resin by addition of trace metal clean 0.1M HNO₃ for 1-2 days, then the supernatant was transferred into a clean fluorocarbon vial and taken to dryness on a hotplate in a clean flow fume hood in a positive pressure clean lab. A PA1 resin blank was taken from a batch of resin directly placed into the eluting 0.1M HNO₃ and henceforward treated as a sample.
Eichrom AG-1x8 resin was cleaned by three batch rinses with 6N trace metal clean HCl for a ~12 hours on a shaker table, followed by multiple washes with distilled water until the pH of the solution was above 4.5. Resin was stored at room temperature in the dark until use.
The residue from the samples and blanks was dissolved in 8 drops of high purity 1.1M HBr. The resin in the column was first cleaned with 6M HCl, equilibrated with 1.1M HBr, and then sample was loaded onto the column. The column was then washed with 1.1M HBr followed by 2M HCl and then eluted with 6M HCl. The samples in a 5 ml Savillex PTFE vial were then taken to dryness on a hotplate in a recirculating filtered air fume hood, and stored sealed until analysis.
Just before analysis, samples were dissolved for several minutes in 10ul concentrated ultrapure HNO₃. Then, an appropriate volume of ultrapure water was added (typically ~400ul) and spiked with an appropriate amount of Tl for mass fractionation correction. IsoProbe multicollector ICPMS Faraday cups were used to collect on 202Hg, 203Tl, 205Tl, 206Pb, 207Pb, and 208Pb. An Isotopx Daly detector with a WARP filter was used to collect on 204Pb+204Hg. This Daly detector is a revised version that eliminates a reflection problem with the electronic circuitry of the previous version. Because the deadtime of the Daly detector varied from day to day, we calibrated deadtime on each day by running a standard with known 206Pb/204Pb at a high 204 count rate. The counter efficiency drifts during the course of a day, so we established that drift by running a standard with known 206Pb/204Pb (and a 204 count rate comparable to the samples) every five samples. Tailing from one Faraday cup to the next was corrected by the 209Bi half-mass method as described by Thirlwall (2001).
Data Processing:
During the isotope ratio analysis, the data were collected in 20 cycles. The isotope ratios were edited for outliers, and averaged with internal standard errors estimated from the multiple cycles. This should be understood as the lower limit to the true error: the reported value cannot be any better than the internal reproducibility of the isotope run. Standard Ocean Data View flags were used (reference all flags at https://www.bodc.ac.uk/data/codes_and_formats/odv_format):
1: Good Value: Good quality data value that is not part of any identified malfunction and has been verified as consistent with real phenomena during the quality control process.
2: Probably Good Value: Data value that is probably consistent with real phenomena but this is unconfirmed or data value forming part of a malfunction that is considered too small to affect the overall quality of the data object of which it is a part. [Used when only one replicate confirmed the reported value.]
3: Probably Bad Value: Data value recognized as unusual during quality control that forms part of a feature that is probably inconsistent with real phenomena.
4: Bad Value: An obviously erroneous data value.
Blank Values and Detection Limits:
Two different methods were used to determined Pb concentrations due to very low Pb concentrations. See Boyle et al. (2020) for more detail. Pb concentrations are already intercalibrated and were included in IDP2017.
Average blanks for the 1.5 mL method: 4.3 – 7.0 (± 1.9 SD) pmol/kg (based on 10 analysis days and 12 blanks per day)
Average blanks for 30 mL method: 0.2 - 3.9 (± 0.9 SD) pmol/kg (based on 20 analysis days and 12 blanks per day)
Blanks: There are three main sources of blanks in this analysis. The major contribution comes from the Nobias Chelate PA1 resin, whereas a minor contribution comes from the Eichrom AG-1x8 resin. A significant but minor contribution also comes from the ICPMS hardware from Pb retained from previous analyses. The latter blank is determined by an "On Peak Zero" measurement on high purity 0.2M HNO₃. We cannot measure the PA1 blank directly because the organic matter released by the resin interferes with the isotopic analysis. So we measure one blank value for PA1 + AG1-x8, and then another one for AG1-x8 alone, determining the PA1 blank by difference.
Because the AG1-x8 blank is so low, we cannot make an accurate measurement of its Pb isotope ratios. So we measure the most intense signal (208Pb) and then estimate the blanks for the other isotopes assuming an isotope composition for "typical U.S. Pb" assumed from the ratio of our U.S.- purchased laboratory Pb standard. Because the PA1 blank is higher, we averaged the isotopic compositions observed from several high-blank runs and assumed these applied to all runs using the same batch of PA1.
All of the signals from our large-volume samples were well above Pb concentration detection limits, but the precision of the measurement degrades relative to the Faraday cup Johnson noise (and Daly ion counter statistics) as the signal becomes weaker. We think that our isotope ratio measurements for 206Pb/207Pb are good to the second decimal place for samples with 1-2 pmol/kg Pb.
Internal Consistency:
Seawater Pb concentration samples were analyzed in triplicate on each analytical day. If at least two of these don’t agree within expectations, they are re-run again in triplicate on another analytical day. Internal lab seawater Pb concentration standards were run every analytical day to ensure long-term consistency.
BCO-DMO Processing:
- replaced '#N/A' with 'nd' as missing data value;
- renamed fields;
- added date/time fields in ISO8601 format.
| File |
|---|
Pb_isotopes.csv (Comma Separated Values (.csv), 46.88 KB) MD5:980959c63adfb77a6895e2d1a9d23035 Primary data file for dataset ID 828221 |
| Parameter | Description | Units |
| Station_ID | Station number | unitless |
| Start_Date_UTC | Start date (UTC) | unitless |
| Start_Time_UTC | Start time (UTC) | unitless |
| Start_ISO_DateTime_UTC | Start date and time (UTC) in ISO8601 format | unitless |
| End_Date_UTC | End date (UTC) | unitless |
| End_Time_UTC | End time (UTC) | unitless |
| End_ISO_DateTime_UTC | End date and time (UTC) in ISO8601 format | unitless |
| Start_Latitude | Start latitude | degrees North |
| Start_Longitude | Start longitude | degrees East |
| End_Latitude | End latitude | degrees North |
| End_Longitude | End longitude | degrees East |
| Event_ID | Event number | unitless |
| Sample_ID | GEOTRACES sample number | unitless |
| Sample_Depth | Sample depth | meters (m) |
| Pb_206_207_D_RATIO_FISH_v3yljd | Atom ratio of given isotopes for dissolved Pb referenced to {NBS981} from seawater samples collected using a trace-metal clean towed surface sampler | unitless |
| SD2_Pb_206_207_D_RATIO_FISH_v3yljd | Two standard deviations of Pb_206_207_D_RATIO_FISH_v3yljd | unitless |
| Flag_Pb_206_207_D_RATIO_FISH_v3yljd | Quality flag for Pb_206_207_D_RATIO_FISH_v3yljd | unitless |
| Pb_206_207_D_RATIO_BOTTLE_anndsd | Atom ratio of given isotopes for dissolved Pb referenced to {NBS981} from seawater samples collected by Niskin bottle or other water sampling bottle | unitless |
| SD2_Pb_206_207_D_RATIO_BOTTLE_anndsd | Two standard deviations of Pb_206_207_D_RATIO_BOTTLE_anndsd | unitless |
| Flag_Pb_206_207_D_RATIO_BOTTLE_anndsd | Quality flag for Pb_206_207_D_RATIO_BOTTLE_anndsd | unitless |
| Pb_208_207_D_RATIO_FISH_z3egzi | Atom ratio of given isotopes for dissolved Pb referenced to {NBS981} from seawater samples collected using a trace-metal clean towed surface sampler | unitless |
| SD2_Pb_208_207_D_RATIO_FISH_z3egzi | Two standard deviations of Pb_208_207_D_RATIO_FISH_z3egzi | unitless |
| Flag_Pb_208_207_D_RATIO_FISH_z3egzi | Quality flag for Pb_208_207_D_RATIO_FISH_z3egzi | unitless |
| Pb_208_207_D_RATIO_BOTTLE_8dtaws | Atom ratio of given isotopes for dissolved Pb referenced to {NBS981} from seawater samples collected by Niskin bottle or other water sampling bottle | unitless |
| SD2_Pb_208_207_D_RATIO_BOTTLE_8dtaws | Two standard deviations of Pb_208_207_D_RATIO_BOTTLE_8dtaws | unitless |
| Flag_Pb_208_207_D_RATIO_BOTTLE_8dtaws | Quality flag for Pb_208_207_D_RATIO_BOTTLE_8dtaws | unitless |
| Pb_206_204_D_RATIO_FISH_xxprlv | Atom ratio of given isotopes for dissolved Pb referenced to {NBS981} from seawater samples collected fusing a trace-metal clean towed surface sampler | unitless |
| SD2_Pb_206_204_D_RATIO_FISH_xxprlv | Two standard deviations of Pb_206_204_D_RATIO_FISH_xxprlv | unitless |
| Flag_Pb_206_204_D_RATIO_FISH_xxprlv | Quality flag for Pb_206_204_D_RATIO_FISH_xxprlv | unitless |
| Pb_206_204_D_RATIO_BOTTLE_anggib | Atom ratio of given isotopes for dissolved Pb referenced to {NBS981} from seawater samples collected by Niskin bottle or other water sampling bottle | unitless |
| SD2_Pb_206_204_D_RATIO_BOTTLE_anggib | Two standard deviations of Pb_206_204_D_RATIO_BOTTLE_anggib | unitless |
| Flag_Pb_206_204_D_RATIO_BOTTLE_anggib | Quality flag for Pb_206_204_D_RATIO_BOTTLE_anggib | unitless |
| Dataset-specific Instrument Name | Isotopx Daly detector |
| Generic Instrument Name | Daly detector |
| Generic Instrument Description | The Daly detector was designed by N.R Daly in the 1960’s. The design uses a conversion dynode to convert incident ions into electrons. It also separates the multiplication electronics away from the ion beam preventing secondary ion production on the multiplication dynodes. |
| Dataset-specific Instrument Name | |
| Generic Instrument Name | GeoFish Towed near-Surface Sampler |
| Generic Instrument Description | The GeoFish towed sampler is a custom designed near surface ( |
| Dataset-specific Instrument Name | 12L GoFlo bottles |
| Generic Instrument Name | GO-FLO Bottle |
| Generic Instrument Description | GO-FLO bottle cast used to collect water samples for pigment, nutrient, plankton, etc. The GO-FLO sampling bottle is specially designed to avoid sample contamination at the surface, internal spring contamination, loss of sample on deck (internal seals), and exchange of water from different depths. |
| Dataset-specific Instrument Name | GV/Micromass IsoProbe multicollector ICPMS |
| Generic Instrument Name | Inductively Coupled Plasma Mass Spectrometer |
| Generic Instrument Description | An ICP Mass Spec is an instrument that passes nebulized samples into an inductively-coupled gas plasma (8-10000 K) where they are atomized and ionized. Ions of specific mass-to-charge ratios are quantified in a quadrupole mass spectrometer. |
TN303
| Website | |
| Platform | R/V Thomas G. Thompson |
| Report | |
| Start Date | 2013-10-25 |
| End Date | 2013-12-20 |
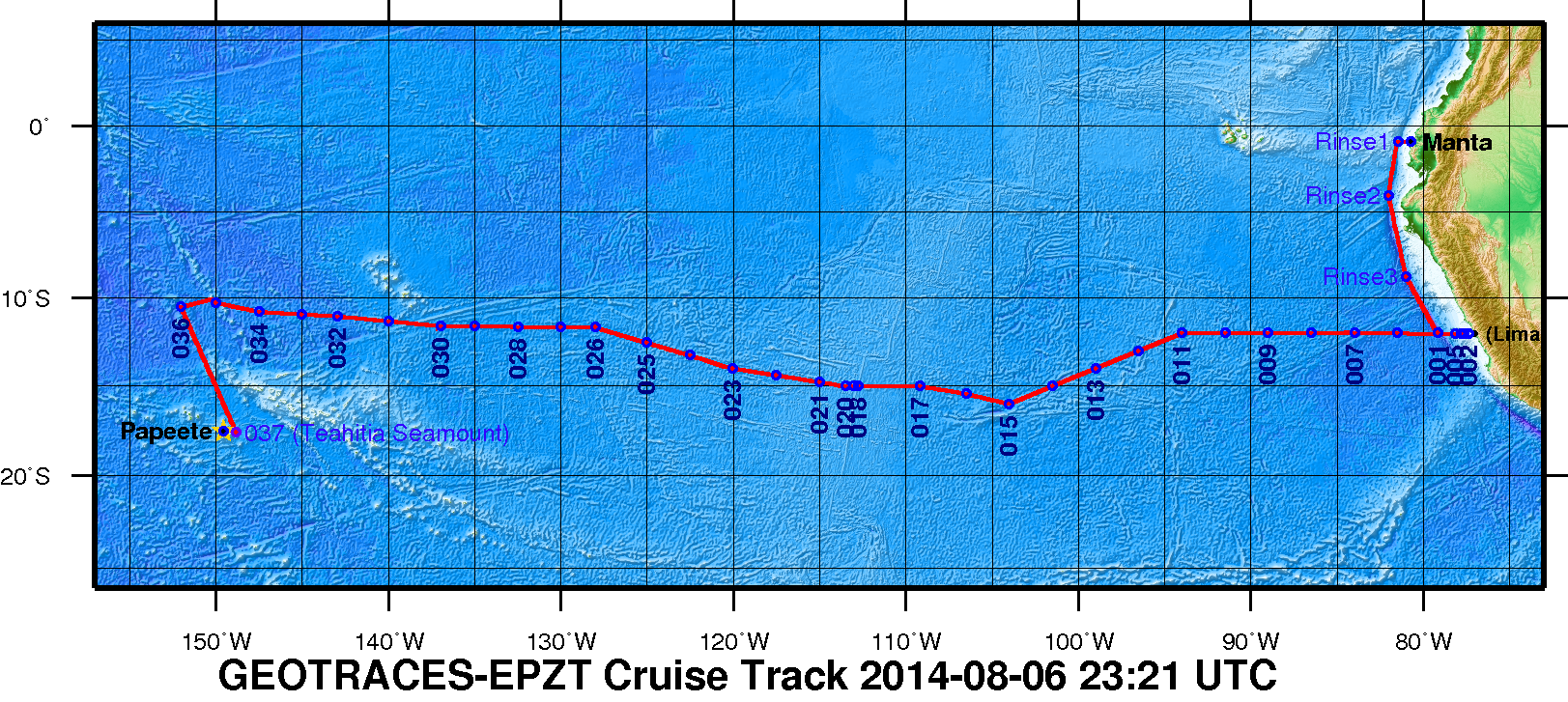
| Description | A zonal transect in the eastern tropical South Pacific (ETSP) from Peru to Tahiti as the second cruise of the U.S.GEOTRACES Program. This Pacific section includes a large area characterized by high rates of primary production and particle export in the eastern boundary associated with the Peru Upwelling, a large oxygen minimum zone that is a major global sink for fixed nitrogen, and a large hydrothermal plume arising from the East Pacific Rise. This particular section was selected as a result of open planning workshops in 2007 and 2008, with a final recommendation made by the U.S.GEOTRACES Steering Committee in 2009. It is the first part of a two-stage plan that will include a meridional section of the Pacific from Tahiti to Alaska as a subsequent expedition.
Figure 1. The 2013 GEOTRACES EPZT Cruise Track. [click on the image to view a larger version]
Additional cruise information is available from the Rolling Deck to Repository (R2R): http://www.rvdata.us/catalog/TN303 |
U.S. GEOTRACES East Pacific Zonal Transect (GP16) (U.S. GEOTRACES EPZT)
From the NSF Award Abstract
The mission of the International GEOTRACES Program (https://www.geotraces.org/), of which the U.S. chemical oceanography research community is a founding member, is "to identify processes and quantify fluxes that control the distributions of key trace elements and isotopes in the ocean, and to establish the sensitivity of these distributions to changing environmental conditions" (GEOTRACES Science Plan, 2006). In the United States, ocean chemists are currently in the process of organizing a zonal transect in the eastern tropical South Pacific (ETSP) from Peru to Tahiti as the second cruise of the U.S.GEOTRACES Program. This Pacific section includes a large area characterized by high rates of primary production and particle export in the eastern boundary associated with the Peru Upwelling, a large oxygen minimum zone that is a major global sink for fixed nitrogen, and a large hydrothermal plume arising from the East Pacific Rise. This particular section was selected as a result of open planning workshops in 2007 and 2008, with a final recommendation made by the U.S.GEOTRACES Steering Committee in 2009. It is the first part of a two-stage plan that will include a meridional section of the Pacific from Tahiti to Alaska as a subsequent expedition.
This award provides funding for management of the U.S.GEOTRACES Pacific campaign to a team of scientists from the University of Southern California, Old Dominion University, and the Woods Hole Oceanographic Institution. The three co-leaders will provide mission leadership, essential support services, and management structure for acquiring the trace elements and isotopes samples listed as core parameters in the International GEOTRACES Science Plan, plus hydrographic and nutrient data needed by participating investigators. With this support from NSF, the management team will (1) plan and coordinate the 52-day Pacific research cruise described above; (2) obtain representative samples for a wide variety of trace metals of interest using conventional CTD/rosette and GEOTRACES Sampling Systems; (3) acquire conventional JGOFS/WOCE-quality hydrographic data (CTD, transmissometer, fluorometer, oxygen sensor, etc) along with discrete samples for salinity, dissolved oxygen (to 1 uM detection limits), plant pigments, redox tracers such as ammonium and nitrite, and dissolved nutrients at micro- and nanomolar levels; (4) ensure that proper QA/QC protocols are followed and reported, as well as fulfilling all GEOTRACES Intercalibration protocols; (5) prepare and deliver all hydrographic-type data to the GEOTRACES Data Center (and US data centers); and (6) coordinate cruise communications between all participating investigators, including preparation of a hydrographic report/publication.
Broader Impacts: The project is part of an international collaborative program that has forged strong partnerships in the intercalibration and implementation phases that are unprecedented in chemical oceanography. The science product of these collective missions will enhance our ability to understand how to interpret the chemical composition of the ocean, and interpret how climate change will affect ocean chemistry. Partnerships include contributions to the infrastructure of developing nations with overlapping interests in the study area, in this case Peru. There is a strong educational component to the program, with many Ph.D. students carrying out thesis research within the program.
Figure 1. The 2013 GEOTRACES EPZT Cruise Track. [click on the image to view a larger version]
Collaborative Research: GEOTRACES Pacific section: Spatial variability of lead concentrations and isotopic compositions in the Eastern Tropical South Pacific (EPZT Pb)
Description from NSF award abstract:
Scientists from Massachusetts Institute of Technology and the University of California, Santa Cruz, plan to measure lead (Pb) concentrations and Pb isotopes (204Pb, 206Pb, 207Pb and 208Pb) in seawater profiles, aerosols, and hydrothermal plume samples collected during the 2013 GEOTRACES cruise from Peru to Tahiti.
Not only will the data significantly add to the limited data set existing for the South Pacific Ocean, results will be used to further our knowledge on the cycling of this element in the marine environment, as well as improve our understanding of anthropogenic inputs of Pb to the region of interest which is important given increases in lead emissions from mining, smelting, and fossil fuel combustion. In addition, data from the East Pacific Rise plume will document whether hydrothermal vent systems contribute to the oceanic Pb budget.
U.S. GEOTRACES (U.S. GEOTRACES)
GEOTRACES is a SCOR sponsored program; and funding for program infrastructure development is provided by the U.S. National Science Foundation.
GEOTRACES gained momentum following a special symposium, S02: Biogeochemical cycling of trace elements and isotopes in the ocean and applications to constrain contemporary marine processes (GEOSECS II), at a 2003 Goldschmidt meeting convened in Japan. The GEOSECS II acronym referred to the Geochemical Ocean Section Studies To determine full water column distributions of selected trace elements and isotopes, including their concentration, chemical speciation, and physical form, along a sufficient number of sections in each ocean basin to establish the principal relationships between these distributions and with more traditional hydrographic parameters;
* To evaluate the sources, sinks, and internal cycling of these species and thereby characterize more completely the physical, chemical and biological processes regulating their distributions, and the sensitivity of these processes to global change; and
* To understand the processes that control the concentrations of geochemical species used for proxies of the past environment, both in the water column and in the substrates that reflect the water column.
GEOTRACES will be global in scope, consisting of ocean sections complemented by regional process studies. Sections and process studies will combine fieldwork, laboratory experiments and modelling. Beyond realizing the scientific objectives identified above, a natural outcome of this work will be to build a community of marine scientists who understand the processes regulating trace element cycles sufficiently well to exploit this knowledge reliably in future interdisciplinary studies.
Expand "Projects" below for information about and data resulting from individual US GEOTRACES research projects.
| Funding Source | Award |
|---|---|
| NSF Division of Ocean Sciences (NSF OCE) |
[ table of contents | back to top ]