Methodology from Spivak, AC and J Ossolinski. 2016. Limited effects of nutrient enrichment on bacterial carbon sources in salt marsh tidal creek sediments. Marine Ecology Progress Series. 544:107-130. 10.3354/meps11587
Sediment samples for organic matter composition were collected by placing a hard plastic sleeve around a polyvinyl chloride (PVC) corer (5 cm diameter x 15 cm deep) and then removing the corer. The plastic sleeve remained in place to maintain the integrity of the sediment column and mark the core location. The top 0.5 cm of each core was collected into pre-combusted vials and frozen (-80 deg C) until analysis.
Lipid biomarker compounds were extracted using a modified Bligh and Dyer (1959) method. Sediment samples were extracted with a chloroform : methylene chloride : phosphate buffer saline mixture (2:1:0.8, v:v:v) using a microwave-accelerated reaction system (MARS6); samples were heated to 80deg C for 10 min with continuous stirring. Following extraction, samples were partitioned and the organic phase was removed. The total lipid extract was concentrated under N2 and samples were separated on silica gel columns by eluting with chloroform, acetone (F1/2), and methanol (F3) (Guckert et al. 1985). The F3 (phospholipids) was dried under N2 and saponified with 0.5 M NaOH at 70 deg C for 4 h. Saponified samples were acidified and extracted three times with hexane. The extract was methylated with acidic methanol (95:5 methanol: HCl) and heated overnight at 70deg C to form fatty acid methyl esters (FAME). Samples were analyzed with an Agilent 7890 gas chromatograph with an effluent split ~70:30 between a 5975C mass spectrometer and a flame ionization detector. Peaks were separated on an Agilent DB-5 ms column (60 m, 0.25 mm inner diameter, 0.25 μm film). FAME concentrations were quantified using methyl heneicosanoate as an internal standard. FAs are designated A:BwC, where A is the number of carbon atoms, B is the number of double bonds, and C is the position of the first double bond from the aliphatic ‘w’ end of the molecule. The prefixes ‘i’ and ‘a’ refer to iso and anteiso methyl branched FAs and indicate whether the methyl group is attached to the penultimate or antepenultimate carbon atoms (Bianchi & Canuel 2011).
Stable carbon isotope ratios of FAMEs (F3) were determined by the WHOI Organic Mass Spectrometry Facility with a Hewlett-Packard 6890 GC interfaced to a DeltaPlus IRMS. Excess 13C was calculated per Eqs. 1 – 3, where samples collected prior to 13C label application were controls and PLFA concentrations were in units of moles m-2.
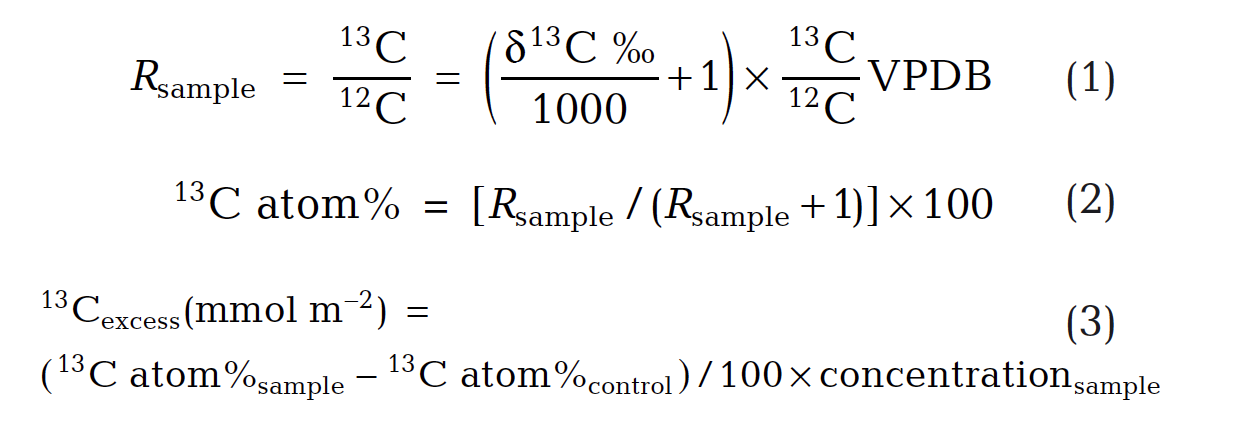
PLFA isotopic values were derived from the isotopic composition of FAMEs and corrected for the d13C of the carbon added during methylation using a mass balance approach. We analyzed total PLFA concentrations as well as concentrations and isotopic composition of compounds and subclasses representing algae (polyunsaturated fatty acids C20:4w6, C20:5w3; PUFA), bacteria (iso- and anteiso- branched C15:0, C17:0; BrFA), sulfate reducing bacteria (10-methyl C16:0), and a combination of algae and microbes (short chain fatty acids C12:0, C14:0; SCFA) (Perry et al. 1979, Kaneda 1991, Volkman et al. 1998). The d13C of PLFA subclasses was calculated as concentrated weighted averages. In order to evaluate the sources of carbon supporting sediment bacteria in the tidal creeks, PLFA isotopic values measured in initial sediment samples (i.e., pre-label application) were corrected for a -3 0/00 fractionation during lipid synthesis (Hayes 2001, Bouillon & Boschker 2006).

 ©2024 Biological and Chemical Oceanography Data Management Office.
©2024 Biological and Chemical Oceanography Data Management Office.
